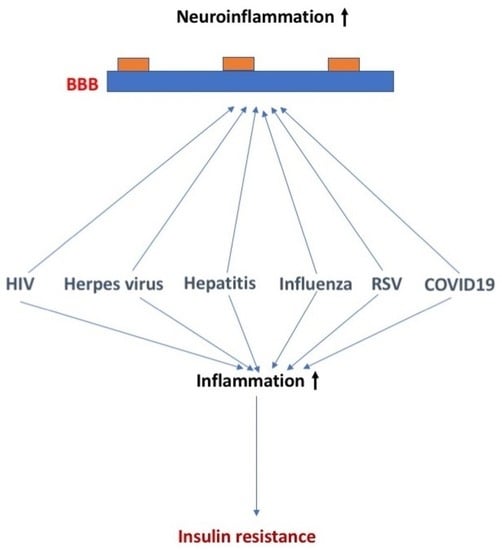
The Effects of Viruses on Insulin Sensitivity and Blood–Brain Barrier Function
Abstract
:1. Introduction
2. Viruses, Insulin Sensitivity, and BBB Function
2.1. HIV, Insulin Sensitivity, and BBB Function
2.2. Herpes Virus, Insulin Sensitivity, and BBB Function
2.3. Hepatitis, Insulin Sensitivity, and BBB Function
2.4. Influenza Virus, Insulin Sensitivity, and BBB Function
2.5. Respiratory Syncytial Virus (RSV), Insulin Sensitivity, and BBB Function
2.6. Coxsackievirus B, Insulin Sensitivity, and BBB Function
2.7. Viral Insulin/IGF-like Peptides (VILPs) and Insulin Sensitivity
2.8. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Insulin Sensitivity, and BBB Function (Figure 6)
3. Simultaneous Exposure to More Than One Virus
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, S.; Clemente-Casares, X.; Zhou, A.C.; Lei, H.; Ahn, J.J.; Chan, Y.T.; Choi, O.; Luck, H.; Woo, M.; Dunn, S.E.; et al. Insulin Receptor-Mediated Stimulation Boosts T Cell Immunity during Inflammation and Infection. Cell Metab. 2018, 28, 922–934.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Li, G. Immune response and blood–brain barrier dysfunction during viral neuroinvasion. Innate Immun. 2020, 27, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Update on acquired immune deficiency syndrome (AIDS)—United States. MMWR Morb. Mortal. Wkly. Rep. 1982, 31, 507–508, 513–514.
- Carr, A.; Samaras, K.; Burton, S.; Law, M.; Freund, J.; Chisholm, D.J.; Cooper, D.A. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. Aids 1998, 12, F51–F58. [Google Scholar] [CrossRef]
- Walli, R.; Herfort, O.; Michl, G.M.; Demant, T.; Jäger, H.; Dieterle, C.; Bogner, J.R.; Landgraf, R.; Goebel, F.D. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. Aids 1998, 12, F167–F173. [Google Scholar] [CrossRef]
- Larson, R.; Capili, B.; Eckert-Norton, M.; Colagreco, J.P.; Anastasi, J.K. Disorders of glucose metabolism in the context of human immunodeficiency virus infection. J. Am. Acad. Nurse Pract. 2006, 18, 92–103. [Google Scholar] [CrossRef]
- Aboud, M.; Elgalib, A.; Kulasegaram, R.; Peters, B. Insulin resistance and HIV infection: A review. Int. J. Clin. Pract. 2007, 61, 463–472. [Google Scholar] [CrossRef]
- Woerle, H.J.; Mariuz, P.R.; Meyer, C.; Reichman, R.C.; Popa, E.M.; Dostou, J.M.; Welle, S.L.; Gerich, J.E. Mechanisms for the deterioration in glucose tolerance associated with HIV protease inhibitor regimens. Diabetes 2003, 52, 918–925. [Google Scholar] [CrossRef] [Green Version]
- Fleischman, A.; Johnsen, S.; Systrom, D.M.; Hrovat, M.; Farrar, C.T.; Frontera, W.; Fitch, K.; Thomas, B.J.; Torriani, M.; Côté, H.C.; et al. Effects of a nucleoside reverse transcriptase inhibitor, stavudine, on glucose disposal and mitochondrial function in muscle of healthy adults. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1666–E1673. [Google Scholar] [CrossRef]
- Spieler, G.; Westfall, A.O.; Long, D.M.; Cherrington, A.; Burkholder, G.A.; Funderburg, N.; Raper, J.L.; Overton, E.T.; Willig, A.L. Trends in diabetes incidence and associated risk factors among people with HIV in the current treatment era. Aids 2022, 36, 1811–1818. [Google Scholar] [CrossRef]
- Milic, J.; Renzetti, S.; Ferrari, D.; Barbieri, S.; Menozzi, M.; Carli, F.; Dolci, G.; Ciusa, G.; Mussini, C.; Calza, S.; et al. Relationship between weight gain and insulin resistance in people living with HIV switching to integrase strand transfer inhibitors-based regimens. Aids 2022, 36, 1643–1653. [Google Scholar] [CrossRef]
- Resnick, L.; Berger, J.; Shapshak, P.; Tourtellotte, W.W. Early penetration of the blood-brain-barrier by HIV. Neurology 1988, 38, 9–14. [Google Scholar] [CrossRef]
- Nottet, H.S.; Persidsky, Y.; Sasseville, V.G.; Nukuna, A.N.; Bock, P.; Zhai, Q.H.; Sharer, L.R.; McComb, R.D.; Swindells, S.; Soderland, C.; et al. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J. Immunol. 1996, 156, 1284–1295. [Google Scholar] [CrossRef]
- Dohgu, S.; Ryerse, J.S.; Robinson, S.M.; Banks, W.A. Human immunodeficiency virus-1 uses the mannose-6-phosphate receptor to cross the blood-brain barrier. PLoS ONE 2012, 7, e39565. [Google Scholar] [CrossRef] [Green Version]
- Persidsky, Y.; Zheng, J.; Miller, D.; Gendelman, H.E. Mononuclear phagocytes mediate blood-brain barrier compromise and neuronal injury during HIV-1-associated dementia. J. Leukoc. Biol. 2000, 68, 413–422. [Google Scholar] [CrossRef]
- Kanmogne, G.D.; Schall, K.; Leibhart, J.; Knipe, B.; Gendelman, H.E.; Persidsky, Y. HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across the blood-brain barrier: Implication for viral neuropathogenesis. J. Cereb. Blood Flow Metab. 2007, 27, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, S.; Castro, V.; Toborek, M. Infection of human pericytes by HIV-1 disrupts the integrity of the blood-brain barrier. J. Cell Mol. Med. 2012, 16, 2950–2957. [Google Scholar] [CrossRef]
- Price, T.O.; Eranki, V.; Banks, W.A.; Ercal, N.; Shah, G.N. Topiramate treatment protects blood-brain barrier pericytes from hyperglycemia-induced oxidative damage in diabetic mice. Endocrinology 2012, 153, 362–372. [Google Scholar] [CrossRef] [Green Version]
- Rom, S.; Gajghate, S.; Winfield, M.; Reichenbach, N.L.; Persidsky, Y. Combination of HIV-1 and Diabetes Enhances Blood Brain Barrier Injury via Effects on Brain Endothelium and Pericytes. Int. J. Mol. Sci. 2020, 21, 13. [Google Scholar] [CrossRef]
- Dohgu, S.; Banks, W.A. Brain pericytes increase the lipopolysaccharide-enhanced transcytosis of HIV-1 free virus across the in vitro blood-brain barrier: Evidence for cytokine-mediated pericyte-endothelial cell cross talk. Fluids Barriers CNS 2013, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- András, I.E.; Leda, A.; Contreras, M.G.; Bertrand, L.; Park, M.; Skowronska, M.; Toborek, M. Extracellular vesicles of the blood-brain barrier: Role in the HIV-1 associated amyloid beta pathology. Mol. Cell Neurosci. 2017, 79, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Woelfle, T.; Linkohr, B.; Waterboer, T.; Thorand, B.; Seissler, J.; Ch’adeau-Hyam, M.; Peters, A. Health impact of seven herpesviruses on (pre)diabetes incidence and HbA1c: Results from the KORA cohort. Diabetologia 2022, 65, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Piras, E.; Madeddu, M.; Palmieri, G.; Angius, F.; Contini, P.; Pompei, R.; Ingianni, A. High Prevalence of Human Herpesvirus 8 Infection in Diabetes Type 2 Patients and Detection of a New Virus Subtype. Adv. Exp. Biol. Med. 2017, 973, 41–51. [Google Scholar]
- Sun, Y.; Pei, W.; Wu, Y.; Yang, Y. An Association of Herpes Simplex Virus Type 1 Infection With Type 2 Diabetes. Diabetes Care 2005, 28, 435–436. [Google Scholar] [CrossRef] [Green Version]
- Clough, D.; Morse, B.; Kucherlapati, R.; Davidson, R. Insulin-induced reactivation of an inactive herpes simplex thymidine kinase gene. Proc. Natl. Acad. Sci. USA 1984, 81, 838–842. [Google Scholar] [CrossRef] [Green Version]
- Rao, P.; Suvas, P.; Jerome, A.; Steinle, J.; Suvas, S. Role of Insulin-Like Growth Factor Binding Protein-3 in the Pathogenesis of Herpes Stromal Keratitis. Immunol. Microbiol. 2020, 61, 46. [Google Scholar] [CrossRef] [Green Version]
- Itzhaki, R. Herpes simplex viris type i and Alzheimer’s disease: Increase evidence for a major role of the virus. Front. Aging Neurosci. 2014, 6, 202. [Google Scholar] [CrossRef]
- Grant, W.B.; Campbell, A.; Itzhaki, R.F.; Savory, J. The significance of environmental factors in the etiology of Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2002, 4, 179–189. [Google Scholar] [CrossRef]
- Itzhaki, R.F.; Faragher, B. Herpes simplex virus and risk of Alzheimer’s disease—Reply. Lancet 1997, 349, 1101–1102. [Google Scholar] [CrossRef]
- Wainberg, M.; Luquez, T.; Koelle, D.; Readhead, B.; Johnston, C.K.; Darvas, M.; Funk, C. The viral hypothesis: How herpesviruses may contribute to Alzheimer’s disease. Mol. Psychiatry 2021, 26, 5476–5480. [Google Scholar] [CrossRef]
- Cairns, D.; Rouleau, N.; Parker, R.; Walsch, K.; Gehrke, L.; Kaplan, D. A 3D human brain–like tissue model of herpes-induced Alzheimer’s disease. Sci. Adv. 2020, 6, eaay8828. [Google Scholar] [CrossRef]
- Linard, M.; Letenneur, L.; Garrigue, I.; Doize, A.; Dartigues, J.-F.; Helmer, C. Interaction between APOE4 and herpes simplex virus type 1 in Alzheimer’s disease. Alzheimer Dement. 2020, 16, 200–208. [Google Scholar] [CrossRef]
- Butgos, J.; Ramirez, C.; Sastre, I.; Valdivieso, F. Effect of apolipoprotein E on the cerebral load of latent herpes simplex virus type 1 DNA. J. Virol. 2006, 80, 5383–5387. [Google Scholar]
- Bourgade, K.; Garneau, H.; Giroux, G.; Le Page, A.; Bocti, C.; Dupuis, G.; Frost, E.H.; Fülöp, T. β-Amyloid peptides display protective activity against the human Alzheimer’s disease-associated herpes simplex virus-1. Biogerontology 2015, 16, 85–98. [Google Scholar] [CrossRef]
- van Dyck, C.; Swanson, C.; Aisen, P.; Bateman, R.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2022, 388, 9–21. [Google Scholar] [CrossRef]
- Tsalenchuck, Y.; Tzuer, T.; Steiner, I.; Panet, A. Different modes of herpes simplex virus type 1 spread in brain and skin tissues. J. Neurosci. 2014, 20, 18–27. [Google Scholar] [CrossRef]
- Buursma, A.; de Vries, E.; Garssen, J.; Kegler, D.; van Waarde, A.; Schirm, J.; Hospers, G.; Mulder, N.; Vaalburg, W.; Klein, H. [18F]FHPG Positron Emission Tomography for Detection of Herpes Simplex Virus (HSV) in Experimental HSV Encephalitis. J. Virol. 2005, 79, 7721–7727. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Qiu, K.; He, Q.; Lei, Q.; Lu, W.-Q. Mechanisms of Blood-Brain Barrier Disruption in Herpes Simplex Encephalitis. J. Neuroimmune Pharmacol. 2019, 14, 157–172. [Google Scholar] [CrossRef]
- Pasieka, T.; Cilloniz, C.; Cartr, V.; Rosaton, P.; Katze, M.; Leiber, D. Functional Genomics Reveals an Essential and Specific Role for Stat1 in Protection of the Central Nervous System following Herpes Simplex Virus Corneal Infection. J. Virol. 2011, 85, 12972–12981. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Huang, C.; Wang, R.; Luo, M.; Lu, W.-Q. Mechanisms of Blood-Brain Barrier Disruption in Herpes Simplex Encephalitis. Front. Mol. Neurosci. 2020, 13, 2. [Google Scholar] [CrossRef] [Green Version]
- Marques, C.; Hu, S.; Sheng, W.; Lokensgard, J. Microglial cells initiate vigorous yet non-protective immune responses during HSV-1 brain infection. Virus Res. 2006, 121, 1–10. [Google Scholar] [CrossRef] [PubMed]
- DeBiasi, R.; HKleinschmidt-DeMasters, B.; Richardson-Burns, S.; Tyler, K. Central nervous system apoptosis in human herpes simplex virus and cytomegalovirus encephalitis. J. Infect. Dis. 2002, 186, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Klein, A. Common herpes virus causes signs of Alzheimer’s disease in brain cells. Scientist 2020, 3282. [Google Scholar]
- Martin, N. The Discovery Of Viral Hepatitis: A Military Perspective. J. R Army Med. Corps 2003, 149, 121–124. [Google Scholar] [CrossRef]
- Castaneda, D.; Gonzalez, A.; Alomari, M.; Tandon, K.; Zervos, X. From hepatitis A to E: A critical review of viral hepatitis. World J. Gastroenterol. 2021, 27, 1691–1715. [Google Scholar] [CrossRef]
- Alzahrani, N. From hepatitis A to E: A critical review of viral hepatitis. Microbiol. Immunol. 2022, 66, 453–491. [Google Scholar] [CrossRef]
- Mehta, S.; Brancati, F.; Strathdee, S. From hepatitis A to E: A critical review of viral hepatitis. Hepatology 2003, 38, 50–56. [Google Scholar] [CrossRef]
- Matsuda, H.; Atsumi, T.; Fujisaku, A.; Shimuzi, C.; Yoshioka, N.; Koike, T. Acute onset of type 1 diabetes accompanied by acute hepatitis C: The potential role of proinflammatory cytokine in the pathogenesis of autoimmune diabetes. Diabetes Res. Clin. Pract. 2007, 75, 357–361. [Google Scholar] [CrossRef]
- Chen, L.; Chou, Y.; Tsai, S.; Hwang, S.; Lee, S. Hepatitis C virus infection-related type 1 diabetes mellitus. Diabet. Med. 2005, 22, 340–343. [Google Scholar] [CrossRef]
- Taylor, R. Insulin resistance and type 2 diabetes. Diabetes 2012, 61, 778–790. [Google Scholar] [CrossRef] [Green Version]
- Negro, F.; Forton, D.; Craxi, A.; Sulkowski, M.; Feld, J.; Manns, M. Extrahepatic morbidity and mortality of chronice hepatitis C. Gastroenterology 2015, 149, 1345–1360. [Google Scholar] [CrossRef] [Green Version]
- Olivieira, L.; De Jesus, R.; Boulhosa, R.S.S.B.; Onofre, T.; Mendes, C.M.C.; Vinhas, L.; Waitzberg, D.L.; Lemaire, D.C.; Cavalcante, L.N.; Lyra, A.C.; et al. Factors associated with insulin resistance in patients witth chronic HCV genotype I infection without obesity or type 2 diabetes. J. Am. Coll. Nutr. 2016, 35, 436–442. [Google Scholar] [CrossRef]
- Patel, S.; Jinjuvadia, R.; Patel, R.; Liangpunsakul, S. Insulin resistance is assocated with significant liver fibrosis in chronic hepatitis C patients: A systematic review and meta-analysis. J. Clin. Gastroenterol. 2016, 50, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Moucari, R.; Asselah, T.; Caxals-Hatem, D.; Voitot, H.; Boyer, N.; Ripault, M.P.; Sobesky, R.; Martinot–Peignoux, M.; Maylin, S.; Nicolas–Chanoine, M.H.; et al. Insulin resistance in chronic hepatitis C: Association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology 2008, 134, 416–423. [Google Scholar] [CrossRef]
- Choi, H.; Soh, J.; Lim, J.; Sim, S.; Leen, S. Associated between dementia and hepatitis B and C virus infection. Medicine 2021, 100, e26476. [Google Scholar] [CrossRef]
- Pazienzaa, V.; Clement, S.; Pugnale, P.; Conzelman, S.; Foti, M.; Mangia, A.; Negro, F. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology 2007, 45, 1164–1171. [Google Scholar] [CrossRef]
- Banerjee, S.; Saito, K.; Sit-Goughoulte, M.; Meyer, K.J.; Ray, R.; Ray, R. Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate-1 and impairs the downstream akt/prtein kinase B signaling pathway for insulin resistance. J. Virol. 2008, 82, 2606–2612. [Google Scholar] [CrossRef] [Green Version]
- Knobler, H.; Schattner, A. TNF-a, chronic hepatitis C and diabetes: A tried. Q. J. Med. 2005, 98, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kasai, D.; Adachi, T.; Deng, L.; Nagano-Fujii, M.; Sada, K.; Ikeda, M.; Kato, N.; Ide, Y.H.; Shoji, I.; Hotta, H. HCV replication suppreses cellular glucose uptake through down-regulation of cell surface expression of glucose transporters. J. Hepatol. 2009, 50, 883–894. [Google Scholar] [CrossRef]
- Bernsmeier, C.; Calabrese, D.; Heim, M.; Duong, H. Hepatitis C virus dysregulates glucose homeostasis by a dual mechanism involving induction of PGC1alpha and dephosphoprylation of FoxO1. J. Viral. Hepat. 2014, 21, 9–18. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Z.; Wang, N.; Guo, M.; Chi, X.; Pan, Y.; Jiang, J.; Niu, J.; Ksimu, S.; Li, J.Z.; et al. Role of HDAC9-FoxxO1 in the trasncriptional program associated with hepatic gluconeogenesis. Sci. Rep. 2017, 7, 6102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gualerzi, A.; Bellan, M.; Smirne, C.; Minh, M.; Rigamonti, C.; Burlone, M.; Bonometti, R.; Bianco, S.; Re, A.; Favretto, S.; et al. Improvement of insulin sensitiivty in diabetic and non diabetic patients with chronic hepatitis C with direct antiviral agents. PLoS ONE 2018, 13, e209216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, N.; Wilson, G.; Murray, J.; Hu, K.; Lewis, A.; Reynolds, G.; Stamataki, Z.; Meredith, L.; Rowe, I.; Luo, G.; et al. Hepatitis C virus infect the endothelial cells of the blood-brain- barrier. Gastroenterology 2012, 142, 634–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martindale, S.; Hurley, R.; Taber, K. Neurobiology of neuroimaging of chronic hepatitis C virus: Implications for neurpsychiatry. Neuropsychiatry 2017, 29, A6–A307. [Google Scholar] [CrossRef]
- Adinolfi, L.; Nevola, R.; Lus, G.; Restivo, L.; Guerrera, B.; Romano, C.; Zampino, R.; Rinaldi, L.; Sellitto, A.; Giordano, M.; et al. Chronic hepatitis C virus infection and neurological and psychiatric disorders: An overview. World J. Gastroenterol. 2015, 21, 2269–2280. [Google Scholar] [CrossRef]
- Dias Barbosa, M.; Zaninott, A.; de Campos Mazo, D.; Guimaraes Pessoa, M.; Souza de Oliveiraa, C.; Carrilho, F.; Farias, A. Hepatitis C virus eradication improves immediate and delayed episodic memory in patients treated with interferoon and ribavirin. BMC Gastroenterol. 2017, 17, 122. [Google Scholar]
- Carter, C. Genetic, Transcriptome, Proteomic, and Epidemiological Evidence for Blood-Brain Barrier Disruption and Polymicrobial Brain Invasion as Determinant Factors in Alzheimer’s Disease. J. Alzheimer’s Dis. Rep. 2017, 1, 125–157. [Google Scholar] [CrossRef]
- Tian, D.; Li, W.; Heffron, C.; Wang, B.; Mahsoub, H.; Sooryanarain, H.; Hassebroek, A.; Clark-Deener, S.; LeRoith, T.; Meng, X.-J. Hepatitis E infects brain microvascular endothelial cells, crosses the blood-brain barrier, and invades the central nervous system. Proc. Natl. Acad. Sci. USA 2022, 119, e2201862119. [Google Scholar] [CrossRef]
- Capua, I.; Mercalli, A.; Romero-Tejeda, A.; Pizzuto, M.S.; Kasloff, S.; Sordi, V.; Marzinotto, I.; Lampasona, V.; Vicenzi, E.; De Battisti, C.; et al. Study of 2009 H1N1 Pandemic Influenza Virus as a Possible Causative Agent of Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 4343–4356. [Google Scholar] [CrossRef] [Green Version]
- Ohno, M.; Sekiya, T.; Nomura, N.; Daito, T.J.; Shingai, M.; Kida, H. Influenza virus infection affects insulin signaling, fatty acid-metabolizing enzyme expressions, and the tricarboxylic acid cycle in mice. Sci. Rep. 2020, 10, 10879. [Google Scholar] [CrossRef]
- Gao, P.; Ji, M.; Liu, X.; Chen, X.; Liu, H.; Li, S.; Jia, B.; Li, C.; Ren, L.; Zhao, X.; et al. Apolipoprotein E mediates cell resistance to influenza virus infection. Sci. Adv. 2022, 8, eabm6668. [Google Scholar] [CrossRef]
- Xu, M.M.; Kang, J.Y.; Ji, S.; Wei, Y.Y.; Wei, S.L.; Ye, J.J.; Wang, Y.G.; Shen, J.L.; Wu, H.M.; Fei, G.H. Melatonin Suppresses Macrophage M1 Polarization and ROS-Mediated Pyroptosis via Activating ApoE/LDLR Pathway in Influenza A-Induced Acute Lung Injury. Oxid. Med. Cell Longev. 2022, 2022, 2520348. [Google Scholar] [CrossRef]
- Cardenas, G.; Soto-Hernandez, J.L.; Diaz-Alba, A.; Ugalde, Y.; Merida-Puga, J.; Rosetti, M.; Sciutto, E. Neurological events related to influenza A (H1N1) pdm09. Influenza Other Respir. Viruses 2014, 8, 339–346. [Google Scholar] [CrossRef]
- Hosseini, S.; Wilk, E.; Michaelsen-Preusse, K.; Gerhauser, I.; Baumgartner, W.; Geffers, R.; Schughart, K.; Korte, M. Long-Term Neuroinflammation Induced by Influenza A Virus Infection and the Impact on Hippocampal Neuron Morphology and Function. J. Neurosci. 2018, 38, 3060–3080. [Google Scholar] [CrossRef] [Green Version]
- Jurgens, H.A.; Amancherla, K.; Johnson, R.W. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J. Neurosci. 2012, 32, 3958–3968. [Google Scholar] [CrossRef]
- Chaves, A.J.; Vergara-Alert, J.; Busquets, N.; Valle, R.; Rivas, R.; Ramis, A.; Darji, A.; Majo, N. Neuroinvasion of the highly pathogenic influenza virus H7N1 is caused by disruption of the blood brain barrier in an avian model. PLoS ONE 2014, 9, e115138. [Google Scholar] [CrossRef] [Green Version]
- Bohmwald, K.; Soto, J.A.; Andrade-Parra, C.; Fernandez-Fierro, A.; Espinoza, J.A.; Rios, M.; Eugenin, E.A.; Gonzalez, P.A.; Opazo, M.C.; Riedel, C.A.; et al. Lung pathology due to hRSV infection impairs blood-brain barrier permeability enabling astrocyte infection and a long-lasting inflammation in the CNS. Brain Behav. Immun. 2021, 91, 159–171. [Google Scholar] [CrossRef]
- Gonzalez, R.N.; Torres-Aviles, F.; Carrasco, P.E.; Salas, P.F.; Perez, B.F. Association of the incidence of type 1 diabetes mellitus with environmental factors in Chile during the period 2000–2007. Rev. Med. Chil. 2013, 141, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, T.; Witso, E.; Tapia, G.; Stene, L.C.; Ronningen, K.S. Self-reported lower respiratory tract infections and development of islet autoimmunity in children with the type 1 diabetes high-risk HLA genotype: The MIDIA study. Diabetes Metab. Res. Rev. 2011, 27, 834–837. [Google Scholar] [CrossRef]
- Thomas, S.; Ouhtit, A.; Al Khatib, H.A.; Eid, A.H.; Mathew, S.; Nasrallah, G.K.; Emara, M.M.; Al Maslamani, M.A.; Yassine, H.M. Burden and disease pathogenesis of influenza and other respiratory viruses in diabetic patients. J. Infect. Public Health 2022, 15, 412–424. [Google Scholar] [CrossRef]
- Beran, J.; Ramirez Villaescusa, A.; Devadiga, R.; Nguyen, L.-A.T.; Gruselle, O.; Pirçon, J.-Y.; Struyf, F.; Devaster, J.-M. Respiratory syncytial virus acute respiratory infections in ≥ 65-year-old adults in long-term care facilities in the Czech Republic. Cent. Eur. J. Public Health 2021, 29, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Pockett, R.D.; Campbell, D.; Carroll, S.; Rajoriya, F.; Adlard, N. A comparison of healthcare resource use for rotavirus and RSV between vulnerable children with co-morbidities and healthy children: A case control study. J. Med. Econ. 2013, 16, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, J.J.; Visseren, F.L.; Bouter, K.P.; Diepersloot, R.J. Infection-induced inflammatory response of adipocytes in vitro. Int. J. Obes. 2008, 32, 892–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastard, J.P.; Jardel, C.; Delattre, J.; Hainque, B.; Bruckert, E.; Oberlin, F. Evidence for a link between adipose tissue interleukin-6 content and serum C-reactive protein concentrations in obese subjects. Circulation 1999, 99, 2221–2222. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, P.; Yang, P.; Zheng, J.; Zhao, D. Peripheral blood microRNAs expression is associated with infant respiratory syncytial virus infection. Oncotarget 2017, 8, 96627–96635. [Google Scholar] [CrossRef]
- Bloodworth, M.H.; Rusznak, M.; Pfister, C.C.; Zhang, J.; Bastarache, L.; Calvillo, S.A.; Chappell, J.D.; Boyd, K.L.; Toki, S.; Newcomb, D.C.; et al. Glucagon-like peptide 1 receptor signaling attenuates respiratory syncytial virus-induced type 2 responses and immunopathology. J. Allergy Clin. Immunol. 2018, 142, 683–687.e12. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, C.D.; Bilawchuk, L.M.; McDonough, J.E.; Jamieson, K.C.; Elawar, F.; Cen, Y.; Duan, W.; Lin, C.; Song, H.; Casanova, J.L.; et al. IGF1R is an entry receptor for respiratory syncytial virus. Nature 2020, 583, 615–619. [Google Scholar] [CrossRef]
- Millichap, J.J.; Wainwright, M.S. Neurological complications of respiratory syncytial virus infection: Case series and review of literature. J. Child. Neurol. 2009, 24, 1499–1503. [Google Scholar] [CrossRef]
- Sweetman, L.L.; Ng, Y.T.; Butler, I.J.; Bodensteiner, J.B. Neurologic complications associated with respiratory syncytial virus. Pediatr. Neurol. 2005, 32, 307–310. [Google Scholar] [CrossRef]
- Kawashima, H.; Ioi, H.; Ushio, M.; Yamanaka, G.; Matsumoto, S.; Nakayama, T. Cerebrospinal fluid analysis in children with seizures from respiratory syncytial virus infection. Scand. J. Infect. Dis. 2009, 41, 228–231. [Google Scholar] [CrossRef]
- Espinoza, J.A.; Bohmwald, K.; Cespedes, P.F.; Gomez, R.S.; Riquelme, S.A.; Cortes, C.M.; Valenzuela, J.A.; Sandoval, R.A.; Pancetti, F.C.; Bueno, S.M.; et al. Impaired learning resulting from respiratory syncytial virus infection. Proc. Natl. Acad. Sci. USA. 2013, 110, 9112–9117. [Google Scholar] [CrossRef] [Green Version]
- Nekoua, M.; Alidjinou, E.; Hober, D. Persistent coxsackievirus B infection and pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 503–516. [Google Scholar] [CrossRef]
- Isaacs, S.; Foskett, D.B.; Maxwell, A.J.; Ward, E.J.; Faulkner, C.L.; Luo, J.Y.; Rawlinson, W.D.; Craig, M.E.; Kim, K.W. Viruses and type 1 diabetes: From enteroviruses to the virome. Microorganisms 2021, 9, 1519. [Google Scholar] [CrossRef]
- Kim, K.; Horton, J.L.; Pang, C.N.I.; Jain, K.; Leung, P.; Isaacs, S.R.; Bull, R.A.; Luciani, F.; Wilkins, M.R.; Catteau, J.; et al. Higher abundance of enterovirus A species in the gut of children with islet autoimmunity. Sci. Rep. 2019, 9, 1749. [Google Scholar] [CrossRef] [Green Version]
- Morse, Z.; Horwitz, M. Virus Infection Is an Instigator of Intestinal Dysbiosis Leading to Type 1 Diabetes. Front. Immunol. 2021, 12, 751337. [Google Scholar] [CrossRef]
- Pinkert, S.; Klingel, K.; Lindig, V.; Dörner, A.; Zeichhardt, H.; Spiller, O.B.; Fechner, H. Virus- host coevolution in a persistently coxsackievirus B3-infected cardiomyocyte cell line. J. Virol. 2011, 85, 13409–13419. [Google Scholar] [CrossRef] [Green Version]
- Walker, L.; von Herrath, M. CD4 T cell differentiation in type 1 diabetes. Clin. Exp. Immunol. 2016, 183, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Engelmann, I.; Alidjinou, E.K.; Bertin, A.; Bossu, J.; Villenet, C.; Figeac, M.; Sane, F.; Hober, D. Persistent coxsackievirus B4 infection induces microRNA dysregulation in human pancreatic cells. Cell. Mol. Life Sci. 2017, 74, 3851–3861. [Google Scholar] [CrossRef]
- Yin, H.; Berg, A.-K.; Westman, J.; Hellerstrom, C.; Frisk, G. Complete nucleotide sequence of a coxsackievirus B-4 strain capable of establishing persistent infection in human pancreatic islet cells: Effects on insulin release, proinsulin synthesis, and cell morphology. J. Med. Virol. 2002, 68, 544–557. [Google Scholar] [CrossRef]
- Bernard, H.; Tejjeiro, A.; Chaves-Perez, A.; Perna, C.; Satish, B.; Novials, A.; Wang, J.; Djouder, N. Coxsackievirus B Type 4 Infection in β Cells Downregulates the Chaperone Prefoldin URI to Induce a MODY4-like Diabetes via Pdx1 Silencing. Cell Rep. Med. 2020, 1, 100125. [Google Scholar] [CrossRef]
- Colli, M.; Paula, F.; Marselli, L.; Marchetti, P.; Roivanen, M.; Eizirik, D.; Op de Beeck, A.J. Coxsackievirus B tailors the unfolded protein response to favour viral amplification in pancreatic β cells. J. Innate Immunol. 2019, 11, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Govic, Y.; Demey, B.; Cassereau, J.; Bahn, Y.-S.; Papon, N. Pathogens infecting the central nervous system. PLOS Pathogens 2022, 18, e1010234. [Google Scholar] [CrossRef]
- Wang, L.; Dong, C.J.; Chen, D.-E.; Song, Z. Coxsackievirus-induced acute neonatal central nervous system disease model. Int. J. Clin. Pathol. 2014, 7, 858–869. [Google Scholar]
- Song, J.; Hu, Y.; Li, H.; Huang, X.; Zheng, H.; Hu, Y.; Wang, J.; Jiang, X.; Li, J.; Yang, Z.; et al. miR-1303 regulates BBB permeability and promotes CNS lesions following CA16 infections by directly targeting MMP9. Emerg. Microbes Infect. 2018, 7, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- H, J.; Haouani, A.; Maatouk, M.; Chekir-Ghedira, L.; Aouni, M.; Fisson, S.; Jaidane, H. Coxsackievirus B4 infection and interneuronal spread in primary cultured neurons. Microb. Pathogen. 2020, 145, 104235. [Google Scholar]
- Moreau, F.; Kirk, N.; Zhang, F.; Gelfanov, V.; List, E.O.; Chrudinová, M.; Venugopal, H.; Lawrence, M.C.; Jimenez, V.; Bosch, F.; et al. Interaction of a viral insulin-like peptide with the IGF-1 receptor produces a natural antagonist. Nat. Commun. 2022, 13, 6700. [Google Scholar] [CrossRef]
- Altindis, E.; Cai, W.; Sakaguchi, M.; Zhang, F.; GuoXiao, W.; Liu, F.; De Meyts, P.; Gelfanov, V.; Pan, H.; DiMarchi, R.; et al. Viral insulin-like peptides activate human insulin and IGF-1 receptor signaling: A paradigm shift for host-microbe interactions. Proc. Natl. Acad. Sci. USA 2018, 115, 2461–2466. [Google Scholar] [CrossRef]
- Norman, J.; Handley, S.A.; Baldridge, M.T.; Droit, L.; Liu, C.Y.; Keller, B.C.; Kambal, A.; Monaco, C.L.; Zhao, G.; Fleshner, P.; et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 2015, 160, 447–460. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Altindis, E.; Kahn, C.; DiMarchi, R.D.; Gelfanov, V. A viral insulin-like peptide is a natural competitive antagonist of the human IGF-1 receptor. Mol. Metab. 2021, 53, 101316. [Google Scholar] [CrossRef]
- Chrudinova, M.; Moreau, F.; Noh, H.; Panikova, T.; Zakova, L.; Friedline, R.; Valenzuela, F.; Kim, J.; Jiracek, J.; Kahn, C.; et al. Characterization of viral insulins reveals white adipose tissue-specific effects in mice. Mol. Metab. 2021, 44, 101121. [Google Scholar] [CrossRef]
- Denson, J.L.; Gillet, A.S.; Zu, Y.; Brown, M.; Pham, T.; Yoshida, Y.; Mauvais-Jarvis, F.; Douglas, I.S.; Moore, M.; Tea, K.; et al. Metabolic Syndrome and Acute Respiratory Distress Syndrome in Hospitalized Patients With COVID-19. JAMA Netw. Open 2021, 4, e2140568. [Google Scholar] [CrossRef]
- Ren, H.; Yang, Y.; Wang, F.; Yan, Y.; Shi, X.; Dong, K.; Yu, X.; Zhang, S. Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19. Cardiovasc. Diabetol. 2020, 19, 58. [Google Scholar] [CrossRef]
- Cromer, S.J.; Colling, C.; Schatoff, D.; Leary, M.; Stamou, M.I.; Selen, D.J.; Putman, M.S.; Wexler, D.J. Newly diagnosed diabetes vs. pre-existing diabetes upon admission for COVID-19: Associated factors, short-term outcomes, and long-term glycemic phenotypes. J. Diabetes Complicat. 2022, 36, 108145. [Google Scholar] [CrossRef]
- Montefusco, L.; Ben Nasr, M.; D’Addio, F.; Loretelli, C.; Rossi, A.; Pastore, I.; Daniele, G.; Abdelsalam, A.; Maestroni, A.; Dell’Acqua, M.; et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 2021, 3, 774–785. [Google Scholar] [CrossRef]
- He, X.; Liu, C.; Peng, J.; Li, Z.; Li, F.; Wang, J.; Hu, A.; Peng, M.; Huang, K.; Fan, D.; et al. COVID-19 induces new-onset insulin resistance and lipid metabolic dysregulation via regulation of secreted metabolic factors. Signal. Transduct. Target. Ther. 2021, 6, 427. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, B.; Chen, D.; Hu, X.; Xu, X.; Shen, W.J.; Hu, C.; Li, J.; Qu, S. COVID-19 May Increase the Risk of Insulin Resistance in Adult Patients Without Diabetes: A 6-Month Prospective Study. Endocr. Pract. 2021, 27, 834–841. [Google Scholar] [CrossRef]
- Boddu, S.; Aurangabadkar, G.; Kuchay, M. New onset diabetes, type 1 diabetes and COVID-19. Diabet Met. Syndr. Clin Res. Rev. 2020, 14, 2211–2217. [Google Scholar] [CrossRef]
- Barron, E.; Bakhai, C.; Kar, P.; Weaver, A.; Bradley, D.; Ismail, H.; Knighton, P.; Holman, N.; Khunti, K.; Sattar, N.; et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: A whole-population study. Lancet Diabetes Endocrinol. 2020, 8, 813–822. [Google Scholar] [CrossRef]
- Keiner, E.S.; Slaughter, J.C.; Datye, K.A.; Cherrington, A.D.; Moore, D.J.; Gregory, J.M. COVID-19 Exacerbates Insulin Resistance During Diabetic Ketoacidosis in Pediatric Patients With Type 1 Diabetes. Diabetes Care 2022, 45, 2406–2411. [Google Scholar] [CrossRef]
- Goldman, S.; Pinhas-Hamiel, O.; Weinberg, A.; Auerbach, A.; German, A.; Haim, A.; Zung, A.; Brener, A.; Strich, D.; Azoulay, E.; et al. Alarming increase in ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the first wave of the COVID-19 pandemic in Israel. Pediatr. Diabetes 2022, 23, 10–18. [Google Scholar] [CrossRef]
- Pietrzak, I.; Michalak, A.; Seget, S.; Bednarska, M.; Beń-Skowronek, I.; Bossowski, A.; Chobot, A.; Dżygało, K.; Głowińska-Olszewska, B.; Górnicka, M.; et al. Diabetic ketoacidosis incidence among children with new-onset type 1 diabetes in Poland and its association with COVID-19 outbreak-Two-year cross-sectional national observation by PolPeDiab Study Group. Pediatr. Diabetes 2022, 23, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, A.; Tascini, C.; Colussi, G.; Peghin, M.; Graziano, E.; De Carlo, C.; Bulfone, L.; Antonello, M.; Sozio, E.; Fabris, M.; et al. Relationship between cytokine release and stress hyperglycemia in patients hospitalized with COVID-19 infection. Front. Med. 2022, 9, 988686. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Rockman-Greenberg, C.; Sareen, N.; Lionetti, V.; Dhingra, S. An insight into the mechanisms of COVID-19, SARS-CoV2 infection severity concerning β-cell survival and cardiovascular conditions in diabetic patients. Mol. Cell Biochem. 2022, 477, 681–1695. [Google Scholar] [CrossRef] [PubMed]
- Govender, N.; Khaliq, O.P.; Moodley, J.; Naicker, T. Insulin resistance in COVID-19 and diabetes. Prim. Care Diabetes 2021, 15, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhang, W.; Li, T.; Yang, G.; Zhu, W.; Chen, N.; Jin, H. Interrelationship between 2019-nCov receptor DPP4 and diabetes mellitus targets based on protein interaction network. Sci. Rep. 2022, 12, 188. [Google Scholar] [CrossRef]
- Shin, J.; Toyoda, S.; Nishitani, S.; Onodera, T.; Fukuda, S.; Kita, S.; Fukuhara, A.; Shimomura, I. SARS-CoV-2 infection impairs the insulin/IGF signaling pathway in the lung, liver, adipose tissue, and pancreatic cells via IRF1. Metabolism 2022, 133, 155236. [Google Scholar] [CrossRef]
- Zangiabadian, M.; Nejadghaderi, S.A.; Zahmatkesh, M.M.; Hajikhani, B.; Mirsaeidi, M.; Nasiri, M.J. The Efficacy and Potential Mechanisms of Metformin in the Treatment of COVID-19 in the Diabetics: A Systematic Review. Front. Endocrinol. 2021, 12, 645194. [Google Scholar] [CrossRef]
- Krasemann, S.; Haferkamp, U.; Pfefferle, S.; Woo, M.S.; Heinrich, F.; Schweizer, M.; Appelt-Menzel, A.; Cubukova, A.; Barenberg, J.; Leu, J.; et al. The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2. Stem Cell Rep. 2022, 17, 307–320. [Google Scholar] [CrossRef]
- Yang, R.C.; Huang, K.; Zhang, H.P.; Li, L.; Zhang, Y.F.; Tan, C.; Chen, H.C.; Jin, M.L.; Wang, X.R. SARS-CoV-2 productively infects human brain microvascular endothelial cells. J. Neuroinflammation 2022, 19, 149. [Google Scholar] [CrossRef]
- Erickson, M.A.; Rhea, E.M.; Knopp, R.C.; Banks, W.A. Interactions of SARS-CoV-2 with the Blood-Brain Barrier. Int. J. Mol. Sci. 2021, 22, 5. [Google Scholar] [CrossRef]
- Haney, J.; Vijakrishnan; Streetley, J.; Dee, K.; Goldfarb, D.; Clark, M.; Mullin, M.; Carter, S.; Bhella, D.; Murcia, P. Coinfection by influenza A virus and respiratory syncytial virus produces hybrid virus particles. Nat. Microbiol. 2022, 7, 1879–1890. [Google Scholar] [CrossRef]
- Gonzalez-Parra, G.; De Ridder, F.; Huntjens, D.; Roymans, D.; Ispas, G.; Dobrovolny, H. A comparison of RSV and influenza in vitro kinetic parameters reveals differences in infecting time. PLoS ONE 2018, 13, e0192645. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Competition between respiratory viruses may hold off a ‘tripledemic’ this winter. Science 2022, 378. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Viral interferecne between repiratory viruses. Emerg. Infect. Dis. 2022, 28, 273–281. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raber, J.; Rhea, E.M.; Banks, W.A. The Effects of Viruses on Insulin Sensitivity and Blood–Brain Barrier Function. Int. J. Mol. Sci. 2023, 24, 2377. https://doi.org/10.3390/ijms24032377
Raber J, Rhea EM, Banks WA. The Effects of Viruses on Insulin Sensitivity and Blood–Brain Barrier Function. International Journal of Molecular Sciences. 2023; 24(3):2377. https://doi.org/10.3390/ijms24032377
Chicago/Turabian StyleRaber, Jacob, Elizabeth M. Rhea, and William A. Banks. 2023. "The Effects of Viruses on Insulin Sensitivity and Blood–Brain Barrier Function" International Journal of Molecular Sciences 24, no. 3: 2377. https://doi.org/10.3390/ijms24032377